 |
.jpg) |
|
Pressure Washers & Steam Cleaners Parts Washers Water Treatment Systems Vapor Steam Cleaning Equipment Floor Care Equipment Industrial Vacuums Ice Blasters Steam Generators & Hot Water Heaters Detergents & Chemicals Custom Design Equipment Accessories / Misc. Equipment Rebuilt Equipment |
Lorchem Learning Center | Cleaning Agent Basics Download the full whitepages here. Go back to the Lorchem Learning Center
Cleaning Agent BasicsArticle courtesy of Kärcher Academy: Basics of Cleaning
|
|
The choice depends on:
|
|
|
Types of dirt:
|
Type of surface:
|
Cleaning Agent Requirements
Cleaning agents must…
1. General:
- Act quickly and easily:
– Have good dirt dissolving and removing effect
– Have a good anti-redeposition effect
– Have a good emulsifying effect
- Be aggressive on the dirt only:
– Be gentle on the skin
- Be easy to use
- Good solubility for powdery products.
- Be odourless or have a pleasant scent.
- Be biologically degradable, safe for humans and the environment.
- Be non-toxic.
2. With respect to the equipment used and objects to be cleaned:
- Not be caustic of oxidising.
- The products must be resistant to high temperatures: Deposits may not occur in the machine or on the cleaned parts.
3. Reasonable selling price
4. There may be further requirements with respect to:
- Viscosity
- Moistening capacity
- Foam development
3. Effect Of Cleaning Agent Components
Reduction of the surface tension (cohesion – adhesion): The surface must be moistened to ensure uniform cleaning. For this purpose, the surface tension must be reduced first. The properties of the surfactant (tenside) molecules (these are the molecules in cleaning agents) accomplish this moistening. This is why they are also called surface-active molecules (more commonly called surfactant molecules).
| Water drop on a smooth surface. No moistening (the water’s surface tension is too high) |
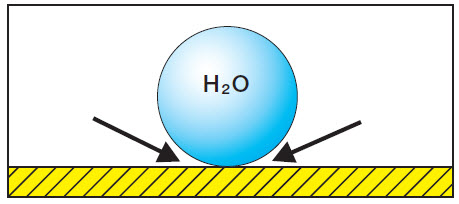 |
|
Low dosage of cleaning agent. |
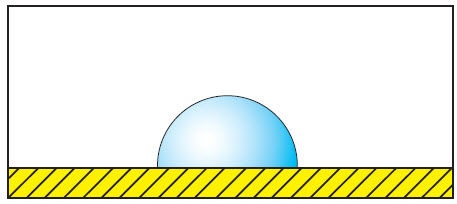 |
| Correct dosage of cleaning agent. Complete moistening (complete reduction of the surface tension). Optimum cleaning. |
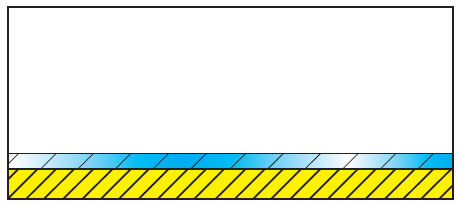 |
Formation Of An Emulsion
An emulsion is the fine distribution of one insoluble liquid in another:
- For example, oil in water;
- Or water in oil;
- Or fat in water (e.g. milk)
The ability of two liquids to emulsify depends on the surface tension of the respective liquids. The emulsifying ability is increased by reducing (or eliminating) the surface tension. This is achieved with the help of an emulsifier. A surfactant can act as an emulsifier.
A cleaning agent is a weak emulsifier if it contains few surfactants and is a strong emulsifier if it contains many surfactants. During cleaning, grease or oil can be detached from the surface as very small droplets and dispersed in the water (emulsion).
Important: The liquids in an emulsion will separate after a certain time. If the cleaning agent is designed in such a way that this separation takes place after the dirt removal but before disposal in the sewers, i.e. in the oil and petrol trap, the wastewater contamination is very low. We call this separation-friendly.
Formation Of A Dispersion
A dispersion is the fine distribution of a solid in a liquid (for example, limescale in water). The ability to keep small particles in suspension is called the anti-redeposition capacity (antiredepositing power). This effect is also produced by the surfactants.
Surfactants (Detergents)
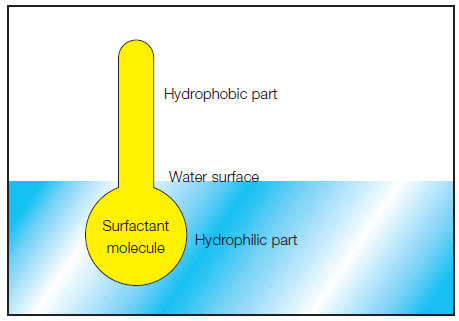 The term “surfactant” is a collective term for different chemical substances. They are divided into the following groups: Detergents, humectants, cleaning agents and rinsing agents.
The term “surfactant” is a collective term for different chemical substances. They are divided into the following groups: Detergents, humectants, cleaning agents and rinsing agents.
- Anionic surfactants
- Cationic surfactants
- Non-ionic surfactants
This classification is based on the charges of the surfactant molecules. A surfactant molecule consists of a hydrophilic and a hydrophobic part.
- The hydrophilic (“water loving”) part wants to remains in the water.
- The hydrophobic (“water fearing”) part is water-repellent.
It therefore wants to get out of the water and prefers to bond with oil and grease. This part is responsible for the emulsifying properties of the surfactant molecule. Due to these two properties, surfactants reduce the interfacial tension.
Effect Of Surfactants
The hydrophobic part of the surfactant tries to get out of the water. The hydrophilic part immerses in the water. As a con sequence of this, all interfaces (water/air, water/wall, water/dirt) are very densely coated with surfactant molecules. This causes a considerable reduction in surface tension. In their efforts to occupy the most interfaces as possible, the surfactant molecules also penetrate areas cannot be accessed by water (between the dirt and the wall). The adhesion between the dirt and surface is thus eliminated. As a result, the dirt is loosened. The dirt particle is then quasi surrounded by surfactants and can be e.g. washed away with a high-pressure jet.
You can see how dirt is suspended in water by the addition of a surfactant-based cleaning agent on the following drawings.
| Water with dirt without cleaning agent. | 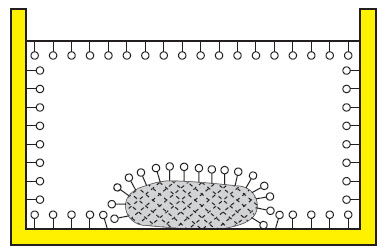 |
| Water with dirt with cleaning agent. | 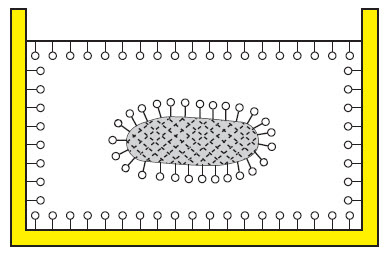 |
| Oil is emulsified. | 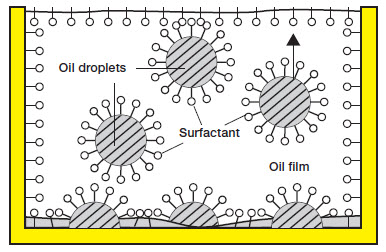 |
Alkalis
Alkalis chemically react with vegetable and animal fats. This chemical reaction causes saponification of the fat. The soap produced by this reaction promotes the effect of the surfactants in the cleaning agent. Alkaline-based agents also enable strongly adhering/bonding dirt to be removed more easily because they swell up. The effect of alkaline-based agents on dirt and grease is also very dependent on the influence of the electrical charges existing between the dirt particles and the surface.
Application: Alkaline-based cleaning agents include degreasers and deep cleaners.
Caution:
- A larger number of materials are sensitive to alkalis, especially when used at high concentrations.
- The strength and quantity of alkalis determine the pH value.
- Alkaline-based agents may have an irritant or caustic effect. You must therefore take the necessary precautions when working with an alkaline-based agent.
Acids
Acids chemically react with mineral (limescale) and oxidised (rust) soiling. The soiling (dirt) is converted by a chemical reaction into water-soluble salts and gases.
Example: CaCO3 + 2 HCl > CaCl2 + CO2 + H2O (Calcium carbonate + hydrochloric acid > calcium chloride + carbon dioxide + water)
Application: Acids are used in sanitary cleaners as surface laitance removers and descalers.
Caution:
- Acids can severely damage materials (limestone, metal)
- Acidic agents may be irritant, caustic or toxic. You must therefore take the necessary precautions when you work with an acidic agent.
Water Hardness Stabilizers
.jpg) Water hardness stabilizers are also called complexing agents. Water contains calcium ions (Ca2+), magnesium ions (Mg2+) and other minerals. Together, these minerals cause so-called water hardness.
Water hardness stabilizers are also called complexing agents. Water contains calcium ions (Ca2+), magnesium ions (Mg2+) and other minerals. Together, these minerals cause so-called water hardness.
Water hardness is expressed in °dH (° German hardness). 1° dH equals 0.179 mmol calcium oxide (millimole per litre water). 0.179 mmol = 10 mg calcium oxide.
Water hardness has adverse effects on the cleaning effect, because Ca2+ and Mg2+ ions are bound to the surfactants. (See diagram to the right: Increasing cleaning agent consumption at higher water hardness). These bonded surfactants are then no longer available for the cleaning effect.
The following substances can be used as builders (or water hardness stabilisers):
- Polyphosphates
- MGDA (methylglycine diacetic acid)
- Triethylene diamine (Trilon), etc.
Polyphosphates have the most advantages in a cleaning agent, but are currently often replaced by MGDA, because these are more environmentally compatible.
Other Additives
Fillers: These are used above all in powder products, solely for price reasons. Fillers are cheap salts, e.g. sodium sulphate.
Inorganic complexing agents: These are used to intensify the cleaning effect and to improve the anti-redepositing power (dispersing ability). Dirt is then held longer in the cleaning liquid and does not redeposit back on the cleaned surface too quickly.
Preservatives: These are substances that ensure that the cleaning agent can be stored for a longer period.
Colourants: These are important for the recognisability of a cleaning agent. They can help to prevent errors in use.
Perfumes: These mainly have a psychological effect when using a product. However, they may also have adverse effects, and therefore these substances must be used with great caution.
- Aggressive agents should not have a very pleasant scent, since this could lead to imprudent use.
- A scent remaining after cleaning is often perceived as being pleasant. However, strongly perfumed products may also be rather undesirable, such as in canteen kitchens or in the food industry.
Bleaching agents: They are used mainly for textile cleaning and have a damaging effect on the laundry (sodium hypochlorite or chlorine bleach).
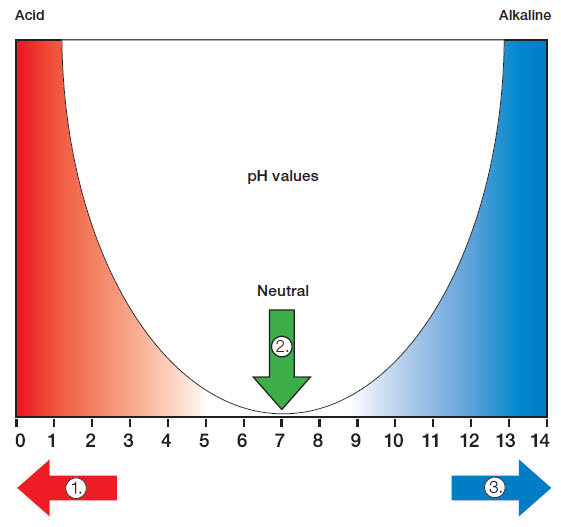
4. The pH Value
The pH value of a cleaning agent indicates the hydrogen ion concentration per litre water. Therefore, only water-based cleaning agents can be classified according to this pH value table.
The pH value can be checked using a pH meter or a pH test strip (litmus paper). |
 |
Why Is The pH Value Important?
The pH value provides information about:
- The use of a cleaning agent
- The substances added (partly)
- Any aggressiveness with respect to materials
- The cleaning effect
- Handling of the cleaning agent (transport, storage, ...)
The pH Value Of The Dirty Water
It is necessary to check the pH value of the dirty water before it can be discharged into the drains. If it is lower than 6.5 or higher than 9 it must be neutralized:
- Acid with a lye (sodium acetate)
- Alkaline with an acid (neutraliser)
Products Without a pH Value
Some cleaning agents do not have a pH value. These products are solvent-based.
5. Solvent-based Cleaning Agents
Solvent-based cleaning agents have a special position among cleaning agents. Organic solvents have a strong fat-dissolving effect. Several kinds of solvents must be mixed with water. hese are then used as additives in water-soluble products.
The most important groups of solvents:
- Alcohol: Methyl alcohol, ethyl alcohol (can be mixed with water).
- Glycol ether,
- Hydrocarbons (frequently used in cold degreasers and heavy-duty cleaners):
– Aromatic; e.g. benzene
– Aliphatic; e.g. cleaner’s solvent (petrol-based), turpentine
– Petroleum
- Chlorinated hydrocarbons: For example, tri- and perchloro ethylene, methylene chloride. Very strong solvents used, for example, in stain removers, paint strippers and degreasers for metals and in electric cleaners. Most chlorinated hydrocarbons are toxic and harmful to the environment. In addition, they have contributed towards the hole in the ozone layer.
6. Water-based Cleaning Agents
Cleaning With An Acidic Agent
The vast majority of cleaning agents are water-based. Dirt containing minerals, e.g. limescale deposits or metal oxides (rust), can only be effectively removed with the help of acidic cleaning agents. Here you must also pay attention to the surface: if it contains calcium carbonate, like marble, then the surface may be damaged.
|
Acids:
|
Used in:
|
Cleaning With a Neutral Agent
|
Advantages:
|
Disadvantages:
|
Use:
|
Cleaning With a Alkaline Agent
|
Advantages:
|
Lyes:
|
Used in:
|
Summary of pH Values
Very alkaline cleaners - pH 14
Strong grease-removing but also caustic properties, dangerous for eyes and skin, attacks light metals (zinc, aluminium) and all kinds of other materials such as paint, linoleum and textiles.
Weaker alkaline cleaners - pH 9
Dirt and grease removal: No or almost no risk of attack, moderately hypoallergenic.
Neutral cleaners - pH 7
Less effective oil and grease removal properties, depending on surfactants used, no limescale or rust compounds, no risk of attacking anything.
Weaker acidic cleaners - pH 6
Limescale and rust removal; grease removal thanks to the influence of detergents and solvents; low risk of attacking metals and skin. Caution with stone containing calcium carbonate!
Very acidic cleaners - pH 1
Powerful limescale and rust removal, skin and eye irritant. There is a risk of metals, paint, glaze and stones containing calcium carbonate/limestone being attacked.
7. Cleaning Agent Limitations
The cleaning agent must never damage the high-pressure cleaner or the surface to be cleaned. Acidic and alkaline agents can cause such damage.
Example
Acid: Attacks metals
Lye: Efflorescence on aluminium surfaces.
When using cleaning agents, environment issues must also be considered (if necessary, neutralise).
Surface damage due to cleaning agents:
| Object Material | Is Attacked By | Note (How To Clean) |
| Iron, Steel | Acidic cleaning agent, free chlorine in disinfectants |
Cleaning agent with inhibitor, phosphatise |
| Aluminium | Alkaline cleaning agent | Clean with acid, use cleaning agents with inhibitors |
| Sheet Metal, Zinc | Very alkaline and very acidic agents | Clean with neutral agent |
| Copper and Alloys | Good cleaning agent resistance | |
| Paint, Varnish | Very alkaline cleaning agent | Clean with mild alkaline agent |
| Rubber | Solvents (e.g. petroleum) | Short reaction time (e.g. preservative removal) |
| Stainless Steel | Free chlorine in disinfectants | Rinse thoroughly |
8. Environmental Aspects
Phosphate
Excessive phosphates in our surface waters represent a risk to the environment. They promote algae growth. This phenomenon is called eutrophication.
Algae release oxygen during the day. However, they need plenty of oxygen at night. This reduces the level of oxygen in the water and there is a large risk of fish mortality. The microorganisms that consume the algae after they have died require oxygen too. This further reduces the water’s oxygen concentrations. This can cause extensive fish mortality especially in the autumn.
- 70% of phosphates come from agriculture (fertilisers)
- 29% come from waste produced by humans
- 1% come from industrial cleaning agents
Problem: In order to prevent more extensive eutrophication, the total phosphorous load must be reduced to less than 1% of the
present day level. Phosphates in cleaning agents are difficult to replace because they are used for several reasons: Water softening, dirt-dissolving and anti-redepositing properties.
Strong Acids and Alkalis
- Represent a risk to drain pipes, sealing materials and sewer materials.
- Can cause a shift in pH of the water. This can endanger plant and animal life in the water (e.g. acidification of surface waters due to acid rain and fish mortality).
The pH values of the discharged water must lie between 6.5 and 9. If this is not the case, it must be neutralised.
Solvents
- Due to their toxicity, chlorinated hydrocarbons, for example tri- or perchloro ethylene, represent an imminent threat (none contained in Kärcher cleaning agents).
- Mineral oil products, such as petroleum or turpentine (white spirit), are highly water polluting. Both mineral oil products and oil-based products must be discharged via an oil separator. There are strict requirements regarding the time required for the separation of a product.
- Cold degreasers are mineral oil-based products, generally mixed with aromatic hydrocarbons. Aromatic hydrocarbons are very good grease cutters, but are toxic.
If too many emulsifying agents are added to the cold degreaser, it is impossible to achieve good separation within the specified time.
Environmental Requirements
Biological degradability:
Most water-based cleaning agents are biologically degradable. However, not only must the detergents be fully degradable; but also the degradation process may not produce or release any toxic intermediate products. Surfactants must be 90% degraded within a given time and under specific conditions. The degradation process for the remaining 10% can take place more slowly, but must actually take place.
Safety
Many cleaning agents can be used safely. Examples are neutral and weak alkaline-based cleaners for daily maintenance cleaning. To prevent allergies, it is also advisable to wear protective gloves when using such products.
However, aggressive agents must be used in some situations in order to clean efficiently.
 |
a) Flammability (Label: flame symbol) Only applies to solvent-based products.
|
 |
b) Toxic ingredients are very rarely found in cleaning agents (Label: Skull and crossbones) |
 |
c) Aggressive to the eyes, skin and mucous membranes – very acidic and alkaline cleaning agents (Label: Test tube with hand: ”Caustic”)
|
It is mandatory to wear personal protective equipment when using such products. For details regarding transport and use, please refer to the corresponding safety data sheets containing the hazardous material and hazardous goods labels.
In the event of skin or eye contact: Rinse with plenty of water and consult a physician.
Download the full whitepages here. Go back to the Lorchem Learning Center
.jpg)